DAIKIN FLUORO-PEDIA Learn about manufacturing, characteristics and applications
of fluorochemical products
-
-
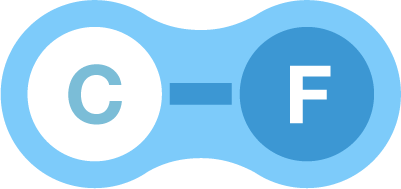



C-F bonds
A fluorine atom
combines with almost all elements easily, and combines with a carbon atom in particular to form the strongest bond (a C-F bond).
C-F bonds have
greater bond energy, and higher electrical stability.
 NEXT
NEXT
-
The energy of a carbon-fluorine bond is greater than the energy of a carbon-hydrogen bond, which is the basic structure of typical materials. This makes a C-F bond more stable, and gives fluorochemicals resistance against almost all energy, such as heat and ultraviolet light, that is exerted on them from the outside.
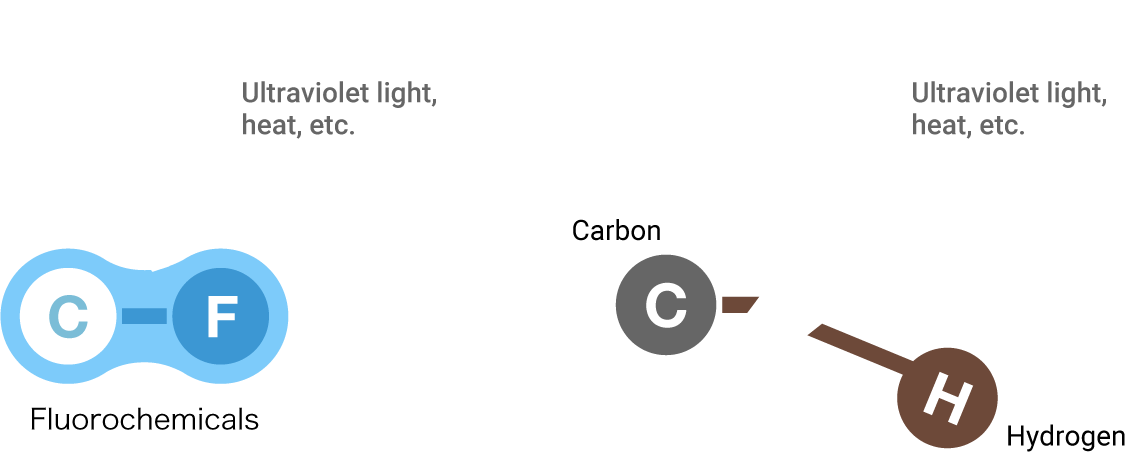






 FluorochemicalsCarbonHydrogen
NEXT
FluorochemicalsCarbonHydrogen
NEXT
-
Fluorochemicals have smaller attraction forces between molecules (the cohesive energy between the molecules) because of the stable C-F bonds.
= The surface tension is low.


Water or oil has a larger cohesive energy between the molecules, and the molecules attract one another.
= The surface tension is high.




Fluorochemicals and water differ in surface tension. When fluorochemicals contact water, water molecules with their higher surface tension attract one another and form a droplet. Thus, the fluorochemical-treated surface repels water.
Fluorochemicals repel oil as well as water.
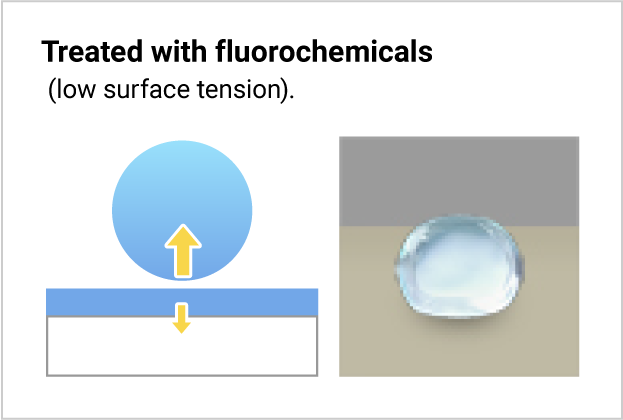
The water is repelled because the attraction force of the water molecules is stronger than the treated base material, and they form a droplet.
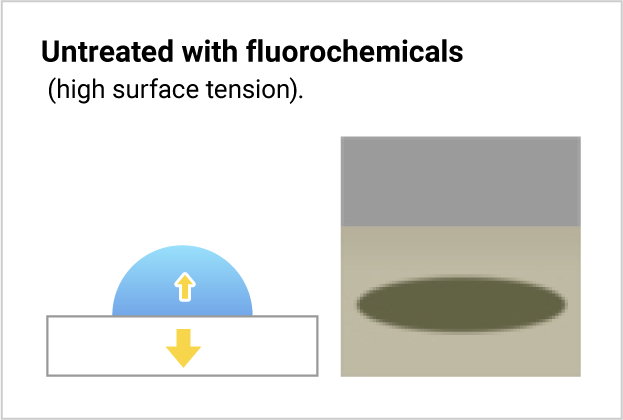
The untreated base material gets wet because it strongly attracts the water.
NEXT
-
Even if you heat fluoroplastics in a microwave, they hardly get warm.
This is because fluoroplastics are electrically stable and less susceptible to high frequencies, due to their symmetrical molecule structure (C2F4)n and the short distance between the carbon and fluorine.
Because fluorochemicals have the lowest dielectric constant and the lowest loss in any plastics, they are utilized for various applications, such as insulating materials inside mobile phones or the wiring in base transceiver stations.




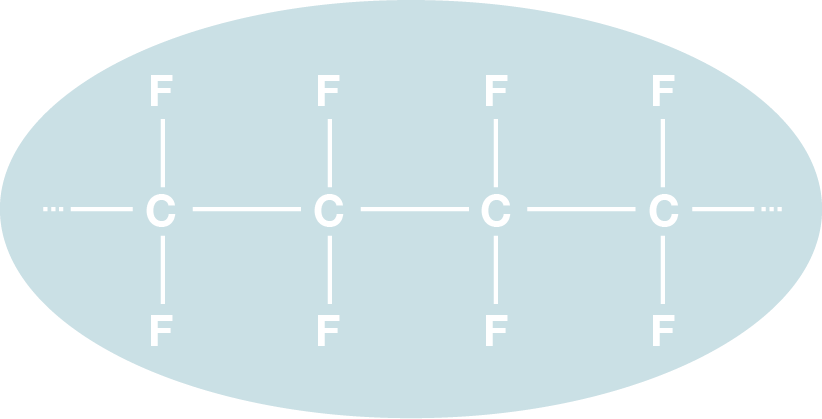
The characteristics of Fluorochemicals-1C-F bonds
The characteristics of Fluorochemicals-2Heat resistance / Chemical resistance / Weatherability
The characteristics of Fluorochemicals-3Water and oil repellency / Lubricity
The characteristics of Fluorochemicals-4Electrical properties
The various applications of fluorochemical products
Heat resistance

Automotive
Crank shaft seal, turbo charger hose, oxgen sensor, under-the hood wires
Chemical resistance
Semiconductor manufacturing
Tubing, fitting, valve ,filter, sealing materials

Oil and gas
O-rings, packer seal
Weatherability

Energy solutions
Photovoltaic solar cell, wind-power generation
The various applications of fluorochemical products
Water and oil repellency

Textile and Fabrics
Textile for apparel, outdoor applications
Lubricity

Home and Living
Cookware
The various applications of fluorochemical products
Electrical properties
Wire and Cable
LAN cable, base station, coaxial cable


